Life Beyond Cancer
It is easy to lose track of what you are fighting for. Chemotherapy, radiation, and surgery are no longer the endgame. Through CAR-T immunotherapy, we believe there is life beyond cancer.

Crossing the Finishing Line
Surgery, radiotherapy, chemotherapy, and precision therapy make up the four established pillars of cancer treatment.
Cancer Immunotherapy has been hailed as the ‘fifth pillar’ of cancer treatment. At the forefront of this ‘fifth pillar’ is CAR-T-Cell Immunotherapy, which is personalized to each patient using their own T-Cells. CAR-T-Cell Immunotherapy aims to improve the immune system’s intrinsic capabilities to identify and attack cancer cells while leaving healthy cells undamaged.
The Fifth Pillar of Cancer Therapy
Cancer Immunotherapy has been hailed as the ‘fifth pillar’ of cancer treatment. At the forefront of this ‘fifth pillar’ is CAR-T cell Immunotherapy, which is personalized to each patient using their own T cells. CAR-T cell Immunotherapy aims to improve the immune system’s intrinsic capabilities to identify and attack cancer cells while leaving healthy cells undamaged.

Surgery
Ancient Times – Present

Radiotherapy
1890s – Present

Chemotherapy
1940s – Present

Precision Therapy
1998s – Present

Immunotherapy
1997s – Present
Efficacy
Chimeric antigen receptor – T cell (CAR-T) therapy, is one of the most promising treatment breakthroughs in recent years. It uses genetically engineered immune T cells to recognize specific proteins on tumor cells. CAR-T clinical trials have shown huge remission rates, of up to 94%, in severe forms of blood cancer. This is particularly impressive considering the fact that most CAR-T clinical trials recruit cancer patients that have not responded to many if not all other available treatments.
When your body does not respond well to conventional therapies, CAR-T provides chance for you to fight back and empowering you with a real chance to rewrite your story.
A living-drug designed to work forever
When T-cells attack harmful cells or viruses, they are programmed to remember them if they attack again. CAR-T-cells are no different. If the process works correctly, they are meant to be on 24/7 duty for the rest of the patient’s life. Theoretically, if cancer cells re-emerge, the CAR-T-cells will recognize them and kill them, even without the patient knowing it. CAR-T cells are designed to have long-lasting effects with one treatment; once you infuse the engineered T-cells, they remain in the body. It is as you are having a living drug in your body for the rest of your life.
The Treatment Process Flow
-
Patient Evaluation and Selection
- Relapse/refractory confirmation
- Referral to panel of CAR-T experts
- Application Assessment and Approval
-
T-Cells Extraction and Preparation
- Leukapheresis (extraction of the Patients T-cells).
- PBMC isolation
- Cryopreservation and shipping for manufacturing
-
Genetic Re-engineering & Expansion
- Patient’s T-cells are engineered with chimeric antigen receptors (CARs).
- The number of the cancer killing CAR-T cells are expanded
- Cryopreservation
- Shipment of the CAR-T cells to the hospital.
-
Infusion
- Pharmacy receipt and product check
- Conditioning therapy is administered
- Patient identity confirmation and product match
- CAR-T Infusion
-
Post Treatment & Recovery
- Strict discharge plan provided to the patient
- Patient monitoring for late-onset adverse effects
- Alternative treatment for non-responses
Possible Side Effects
Cytokine Storm
Potentially life-threatening condition that results from the pathologic over-activation of T-Cells. It is an acute systemic inflammatory syndrome characterized by fever and multiple organ dysfunction.
Cytopenia
Blood consists of red blood cells, which carry oxygen and nutrients around the body, and white blood cells, which fight infection. Cytopenia occurs when the levels of one of these types of blood cells falls abnormally low.
B-Cell Aplasia
B-Cell aplasia occurs when anti-CD19 CAR-T-Cells kill normal B lymphocytes that express CD19. These patients are typically at high risk of developing infections, because of their hypogammaglobulinemia. However, this can be treated with intravenous immunoglobulin (IVIG) replacement therapy.
Neurotoxicity
Damage to the brain or peripheral nervous system caused by exposure to natural or man-made toxic substances. These toxins can alter the activity of the nervous system in ways that can disrupt or kill nerves.
Access to CAR-T Therapy
Patients with an immediately life-threatening condition or serious disease may be granted an expanded access pathway for CAR-T-Cell or other immunotherapies, as provided by the USFDA and regulatory bodies in other countries, under their respective provisions for compassionate use of investigational treatment outside of clinical trials when no comparable or satisfactory alternative therapy options are available.
All treatments with CAR-T products prepared and supplied by our group have been approved by the Ministry of Health of Malaysia and the ministries of health of all other countries where we have supplied the CAR-T products.
Eligibility Criteria
Patients eligible for CAR-T Cell Immunotherapy must meet the following criteria:
- Patients must be well enough to undertake leukapheresis and receive CAR-T therapy.
- The tumor must express the appropriate marker (for example CD19, or CD22).
- The Eastern Cooperative Oncology Group (ECOG) performance status is 0 or 1.
- No disease complications or chemo-toxicity, such as, infection, active GVHD, hyperleukocytosis, severe extra medullary disease.
- The last dose of chemotherapy and/or steroid is at least 2 weeks prior to leukapheresis; when in doubt, a T-Cell activation test shall be performed.
- Patient’s tumor burden is as low as possible. In patients with uncontrolled or accelerating tumor burden, bridging chemotherapy should be performed first (apheresis can be done prior to commencing the chemotherapy).
If any of the above requirements are not met, there is an option for the patient to freeze their T-Cells for future use once treatment can proceed.
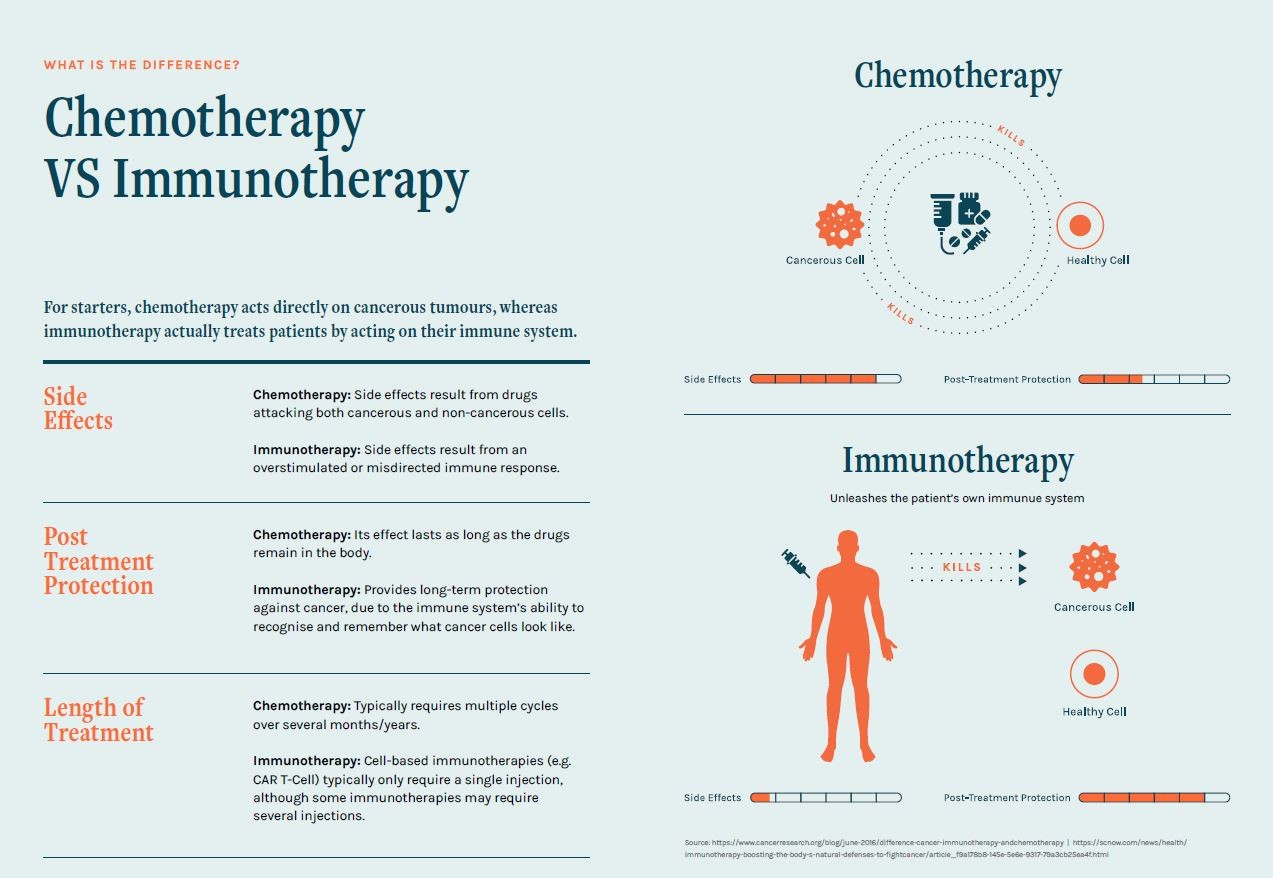
Chemo VS Immunotherapy
Comprehensive Support
- Patient Educational Materials
- Doctor’s Educational Materials
- Free Pre-treatment Assessment
- Comprehensive Auxiliary support for treatments in Malaysia
- Post Treatment Monitoring
Additional Reading
Presentations
Scientific Publications
Vamos life-cell therapies
The Vamos group of companies is engaged in life-cell therapies since 2016. We provide cutting-edge biotechnology products to both hospitals and patients seeking the most effective cancer treatments. Our suite of T-cell based immunotherapies and services are highly accurate, personalized, and focused on providing the most effective treatments based on highest quality standards and at the lowest cost possible.
Vamos Services
Our group provides the following services:
- Production and supply of autologous CAR-T cells for CAR-T treatments provided by hospitals in Malaysia and internationally.
- Medical tourism and auxiliary services related to hosting of international patients taking CAR-T treatments in Malaysia.
Facts about Vamos
- Our group is the only provider of CAR-T therapy in Malaysia for blood cancers.
- Our CAR-T therapy is approved by the Malaysian Ministry of Health.
- All patients that participated in the clinical trial achieved complete remission are live a life free of cancer (with exception of 3 patients that passed away before receiving the actual CAR-T treatment).
- 6 of the top hospitals in Malaysia provide CAR-T treatments with our products.
- So far, we have treated more than 100 number of patients with stage 4 cancer, and the success rate of our CAR-T therapy is over 90%.
- Up to date, all patients treated with our CAR-T therapy have been treated successfully and without experienced any severe side effects.
- The quality of our CAR-T therapy is second to none, while the cost of our therapy is possibly the lowest cost in healthcare worldwide.
- In addition to CAR-T therapy (for both blood and solid cancers), we also provide DC therapy, CIK therapy, and DC-CIK therapy for solid cancers. Together with our clinical trial partner hospital, our group has conducted more than 2,000 DC, CIK & DC-CIK treatments.

References
- Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the Children’s Oncology Group. J Clin Oncol 2012; 30: 1663–69
- Allemani C, Weir HK, Carreira H, et al, and the CONCORD Working Group. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries. Lancet 2014; published online Nov 26. http://dx.doi.org/10.1016/
S0140-6736(14)62038-9 - Nguyen K, Devidas M, Cheng SC, et al. Factors influencing survival after relapse from ALL: a Children’s Oncology Group study AALL01P2. Leukemia 2008; 22: 2142-50
- Raetz EA, Bhatla T. Where do we stand in the treatment of relapsed acute lymphoblastic leukemia? Hematology Am Soc Hematol Educ Program 2012; 2012: 129-36Fielding AK, Richards SM, Chopra R, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood 2007;109:944-50
- Forman SJ, Rowe JM. Myth of second remission of AL in adult, Blood 2013
- Abboud MR, Ghanem K, Muwakkit S. Acute lymphoblastic leukemia in low and middle-income countries: disease characteristics and treatment results. Curr Opin Oncol. 2014 Nov;26(6):650-5
- Caniza MA, Odio C, Mukkada S, Gonzalez M, Ceppi F, Chaisavaneeyakorn S, Apiwattanakul N, Howard SC, Conter V, Bonilla M. Infectious complications in children with acute lymphoblastic leukemia treated in low-middle-income countries. Expert Rev Hematol. 2015 Oct;8(5):627-45
- Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 2018;378:439-48
- Park JH, Rivière I, Gonen M, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med 2018;378:449-59.
- Jaini MD, Davilai ML. Emerging Principles from the Clinical Application of Chimeric Antigen Receptor T Cell Therapies for B Cell Malignancies. Stem Cells 2017; doi: 10.1002/stem.2715
- Saad S. Kenderian, David L. Porter, Saar Gill, Chimeric Antigen Receptor T Cells and Hematopoietic Cell Transplantation: How Not to Put the CART before the Horse, Biol Blood Marrow Transplant 2017; 23: 235–246
- Bru¨ggemann M, Raff T, Kneba M. Has MRD monitoring superseded other prognostic factors in adult ALL? Blood. 2012;120(23):4470-4481.
- Lee, D. W. et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124, 188–195 (2014)
- Neelapu SS, Tummala S, Kebriaei P, Wierda W, et al. Chimeric antigen receptor T-cell therapy- assessment and management of toxicities. Nature Review Clin Oncology 2017 doi:10.1038/nrclinonc.2017.148
