- According to the World Health Organization, the Words Bank and the UN, the Antibacterial Resistance (ABR) is not only the World’s number 1 health problem with devastating effect on health, human life and economy, but it is even bigger treat to humanity than terrorism and global warming.
- In 2018, 700,000 people died as a result of infections caused by bacteria resistant to antibiotics.
- A failure to address the problem of antibiotic resistance will result in an estimated 10 million deaths each year globally by 2050 and a cost of 85 trillion USD in loss productivity, and a catastrophic damage to the global economy.
Useful Links:
- Crises of Antimicrobial Resistance in China, Now and in the Future
- When drugs do not work! Antibiotic Resistance as a Global Development-Problem.
- The end of the golden era of antibiotics
- Antimicrobial resistance and sustainable development, a planetary treat (UN)
- In the era of AMR, Tuberculosis is a priority
Vamos Biotech is the first company in the world to successfully complete a clinical trial and register a medical product (medical device class 2 b) that can efficiently treat large spectrum of antibiotic resistant infections, both gram-positive and gram-negative bacteria. Entomix® is a natural peptide complex comprising of four (4) groups of antibiotic peptides: defensins, cecropins, diptericin’s and proline-reach peptides. It also includes anti-viral and anti-fungal components.
Antimicrobal Activity
AMPs mode of action and activity spectrum
Defensins |
Cell wall disruption |
G+ bacteria fungi |
|---|---|---|
| Diptericins | G- bacteria | |
| Cecropins | G- bacteria | |
| P-peptides | Blocking of protein and DNA synthesis | G+/-bacteria |
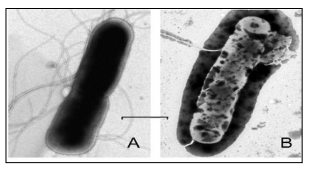

The efficacy of the first FLIP7 product – Entomix® was proven in a clinical trial in Russia, that was conducted in 3 leading hospitals. The clinical trial was conducted under a medical device class 2b classification; the result is GOLD standard in all tests.
Link to the website of the medical registry:
(http://reestrinform.ru/reestr-clinicheskikh-issledovaniy/id-5405.html)
Click here to access the official results from the clinical trial:
The efficacy of the first FLIP7 product – Entomix® was proven in a clinical trial in Russia, that was conducted in 3 leading hospitals. The clinical trial was conducted under a medical device class 2b classification; the result is GOLD standard in all tests.
Name of medical device:
Hydrogel coating to suppress the development of bacterial biofilms according to TU 21.20.23-006-72500079-2017
End of clinical trial: December 2019
Results:
The clinical trial of Entomix® clearly established Entomix® as a GOLD STANDARD in the treatments of:
- diabetic foot ulcer pyoderma,
- severe burns
- post-surgery wound infections
- Antibiotic Resistant (ABR) bacterial infections.
Product Registration:
The product registration of Entomix® in Russia completed successfully in December 2020, and we already received market authorization from the Russian ministry of health.
Hydrogel coating to inhibit the development of bacterial biofilms
RF Patent No. 2447896 “Antimicrobial substance”
RF Patent No. 2664708 “Method of destruction and prevention of the formation of bacterial biofilms by a complex of antimicrobial peptides of insects”
RU number: RZN 2020/12582 dated 17.11.2020
cleans wounds from bacteria
promotes healing
reduces the incidence of infectious complications
increases the effectiveness of antibiotic treatment
contains 4 classes of natural antimicrobial peptides (defensins, cecropins, diptericins and proline-rich peptides)

synthesized by the immune system of insects, purified using the original biosynthesis technology
has no analogues in the world
protected by a patent
Hypersensitive to FLIP ® 7 bacteria
Enterobacteriacea
Escherichia coli
Klebsiella pneumoniae
Salmonella typhimurium
Enterobacter cloacae
Moraxellacea
Acinetobacter baumannii
Bacillacea
bacillus subtilis
bacillus megaterium
Coccacea
Staphylococcus aureus
Micrococcus luteus
Moderately sensitive FLIP ® 7 bacteria
Pseudomonadacea
Pseudomonas aeruginosa
Corynebacteriacea
Listeria mocytogenes
does not lead to the formation of bacterial resistance
acts on both sensitive and antibiotic-resistant bacteria
contributes to the maintenance of normal microflora
powerful antibiotic synergist
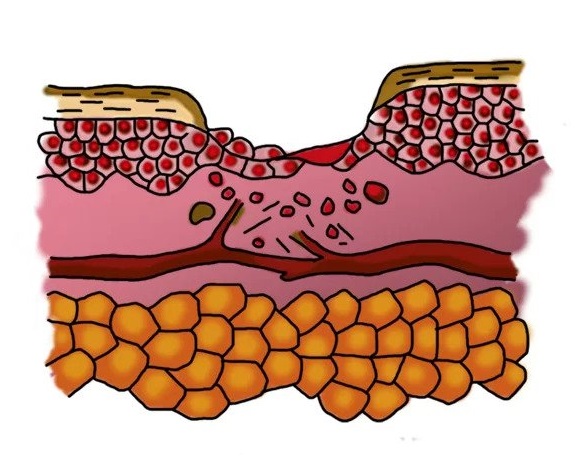
How ENTOMIX ® works in a wound with biofilm
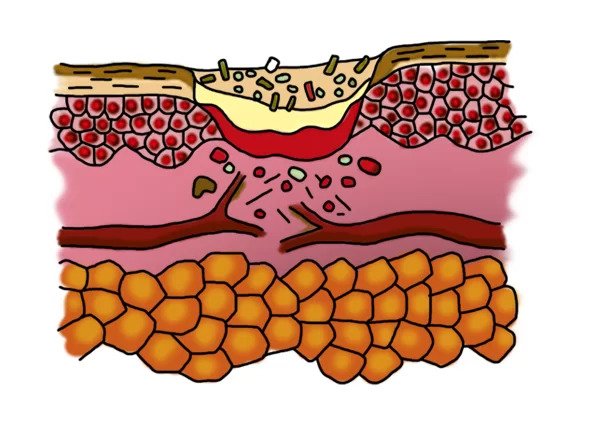
Inflammation phase with biofilm
- formation of a zone of necrosis
- biofilm formation by bacteria
- The “perforated” bacteria dies
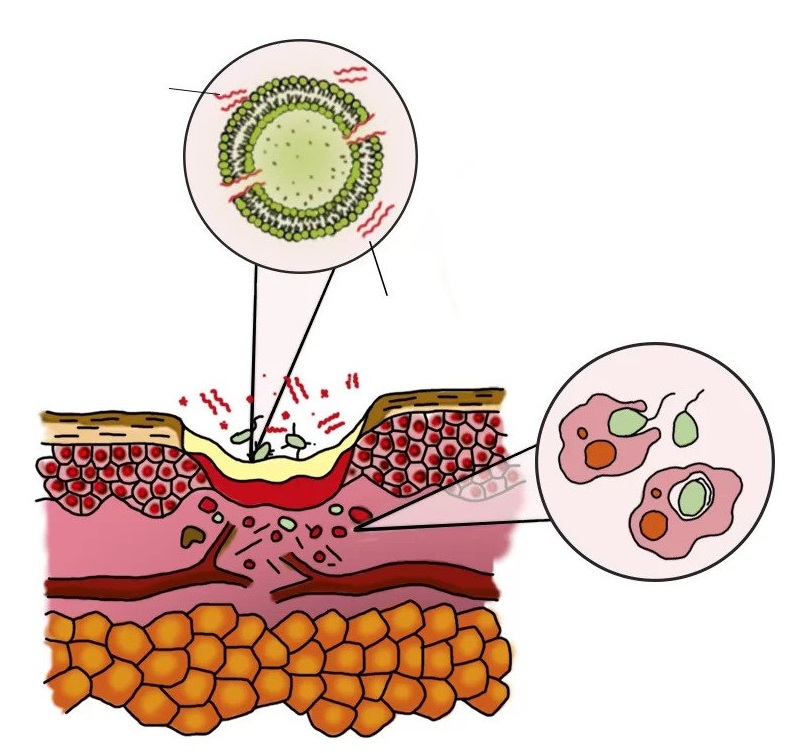
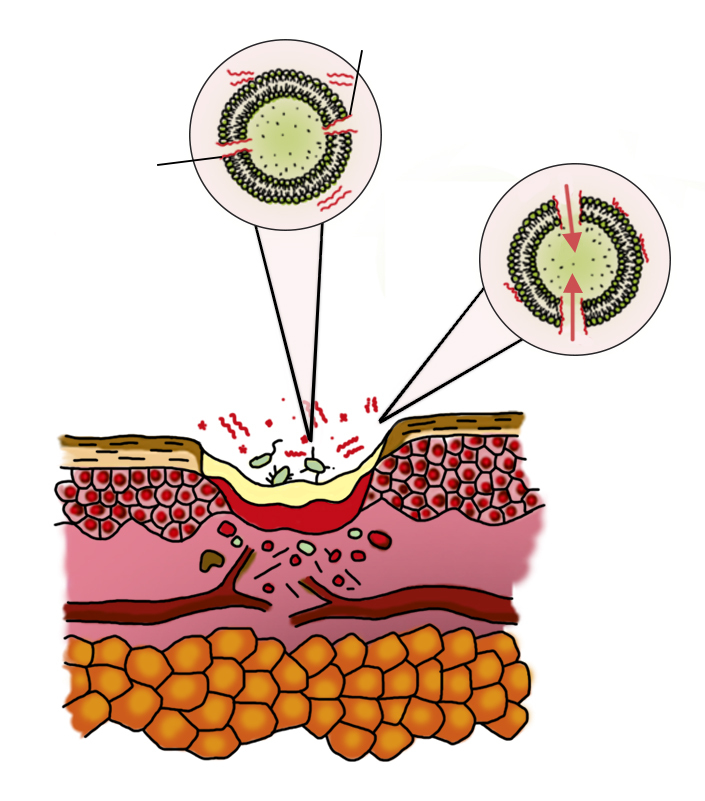
Purification phase
- antimicrobial peptides FLIP ® 7 “stick” to bacteria and are embedded in its membrane
- The “perforated” bacteria dies
- the biofilm is destroyed, the bacteria pass into a free floating state
- migration of phagocytes to the area of inflammation
- cleaning and sterilization of the wound by phagocytes
FLIP ® 7
FLIP ® 7
Regeneration phase
- formation of granulation tissue, blood vessels
- epithelialization
wound surface
phagocytes (cells of the immune system)
blood vessels
epithelial cells
separately floating bacteria
dormant bacteria
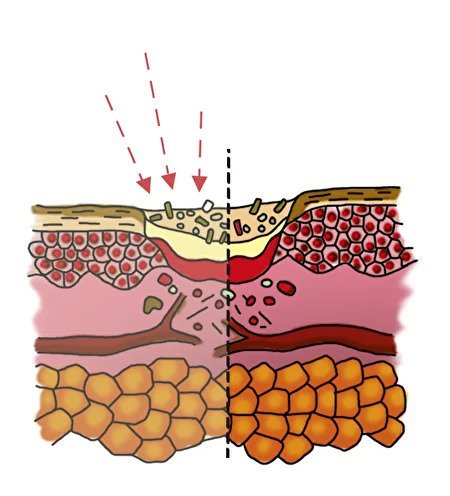
Inflammation phase with biofilm
- formation of a zone of necrosis
- biofilm formation by bacteria
- the biofilm surrounding the bacteria makes it difficult for the antibiotic to come into contact with the bacteria in the wound
A
A
A
Purification phase
- the biofilm is destroyed, the bacteria pass into a free floating state
- antimicrobial peptides FLIP ® 7 “adhere” to bacteria, integrate into its membrane and form a channel
- through a channel in a bacterial cell, the antibiotic enters the bacterium more easily and kills it
FLIP ® 7
FLIP ® 7
A
A
A
A
A

Regeneration phase
- formation of granulation tissue, blood vessels
- epithelialization
wound surface
phagocytes (cells of the immune system)
blood vessels
epithelial cells
separately floating bacteria
dormant bacteria
antibiotic
The ENTOMIX ® medical device is recommended for use in surgery

Provides moist wound management without obstructing aeration
Easily applied to the wound surface
Reduces the concentration of toxins of exogenous and endogenous origin in the damaged area
Prevents the adhesion of the dressing
Reduces the incidence of infectious complications (by accelerating the time of cleansing, regeneration, and final epithelialization of lesions)
Improves aesthetic healing results
Biosurgery or Maggots Debridement Therapy (“MDT”) is a medical technology for the treatment of complex and neglected infected wounds using “surgical larvae” (FDA K033391). In recent years, it has gained popularity in many countries, including the USA, Japan, and EU countries.
The essence of this technology is the placement of “surgical larvae” into the infected wound of the patient, which, in order to protect themselves from the surrounding bacteria, begin to release a complex of antimicrobial peptides into the wound, which in turn leads to the cleansing of the patient’s wound from bacteria.
This complex with such powerful antibacterial properties was formed in larvae over millions of years of evolution. Now this is the “weapon” of larvae against bacteria in the struggle for the same habitat.
Our Scientists managed to isolate this antibacterial complex and study its safety and efficacy in detailed. We named this peptide FLIP® 7 (Fly Larvae Immune Peptides), we used it to create the Entomix® medical device, we conducted and finished a clinical trial and received market authorization in the Russian Federation in the end of 2020.
Flip7 includes 15 antimicrobial peptides peptides from 4 classes: defensins, proline-rich peptides and cecropin dipteritsiny. Each family of antimicrobial peptides is structurally and functionally characterized and has its own mechanism of action.
The use of MDT is associated with many technical difficulties, requires hospitalization of the patient in a specialized medical institution, and is often poorly tolerated by patients.

Entomix® perfectly replaces the complex, time-consuming, and expensive Maggots Debridement Therapy with modern, simple, and low-cost technology for the treatment of infected wounds.
Historical fact
Even during the Crimean War, people saved limbs from gangrene and amputation by planting fly larvae in the wound, which ate necrotic tissue and cleaned the wound, secreting an antimicrobial secret into it. It was the traditional international method of treating infected wounds in the field. The first doctor to apply bio-surgery in Russia was surgeon N.I. Pirogov.
The complex of antimicrobial peptides FLIP ® 7 was discovered and described by the Soviet and Russian scientist-entomologist, Doctor of Biological Sciences and the founder of the company “Allopharm” – Sergei Ivanovich Chernysh.
Before applying the gel, the wound must be treated, if necessary, cleaned with a sterile napkin from necrotic elements to ensure tight contact of the gel components with the wound surface.

ENTOMIX ® should be applied directly to the damaged surface with a thin layer (up to 1 mm) at least 1 time per day.
3 minutes after the application of the ENTOMIX ® hydrogel, the wound can be closed with a secondary dressing (in case of burns, diabetic ulcers), which does not contain strong oxidants, proteolytic enzymes or cytotoxic antiseptics.
The term of application of the coating of the hydrogel ENTOMIX ® is during the period necessary for the healing of the wound surface and removal of the symptoms of inflammation of the damaged area of the skin.
| Document name |
|---|
| Entomix® Instructions for use, English |
| Entomix® Instructions for use, Russian |
| Entomix® Instructions for use, Chinese |
one hour is enough for a biofilm to form.
90% of all chronic wounds contain a biofilm
As the biofilm progresses through these stages, it becomes increasingly difficult for health care practitioners to remove it and for the patient’s immune system to fight it effectively.
after 100 hours, the antibiotics start losing efficacy.
In a clean wound
- migration of phagocytes to the area of inflammation
- cleaning and sterilization of the wound by phagocytes
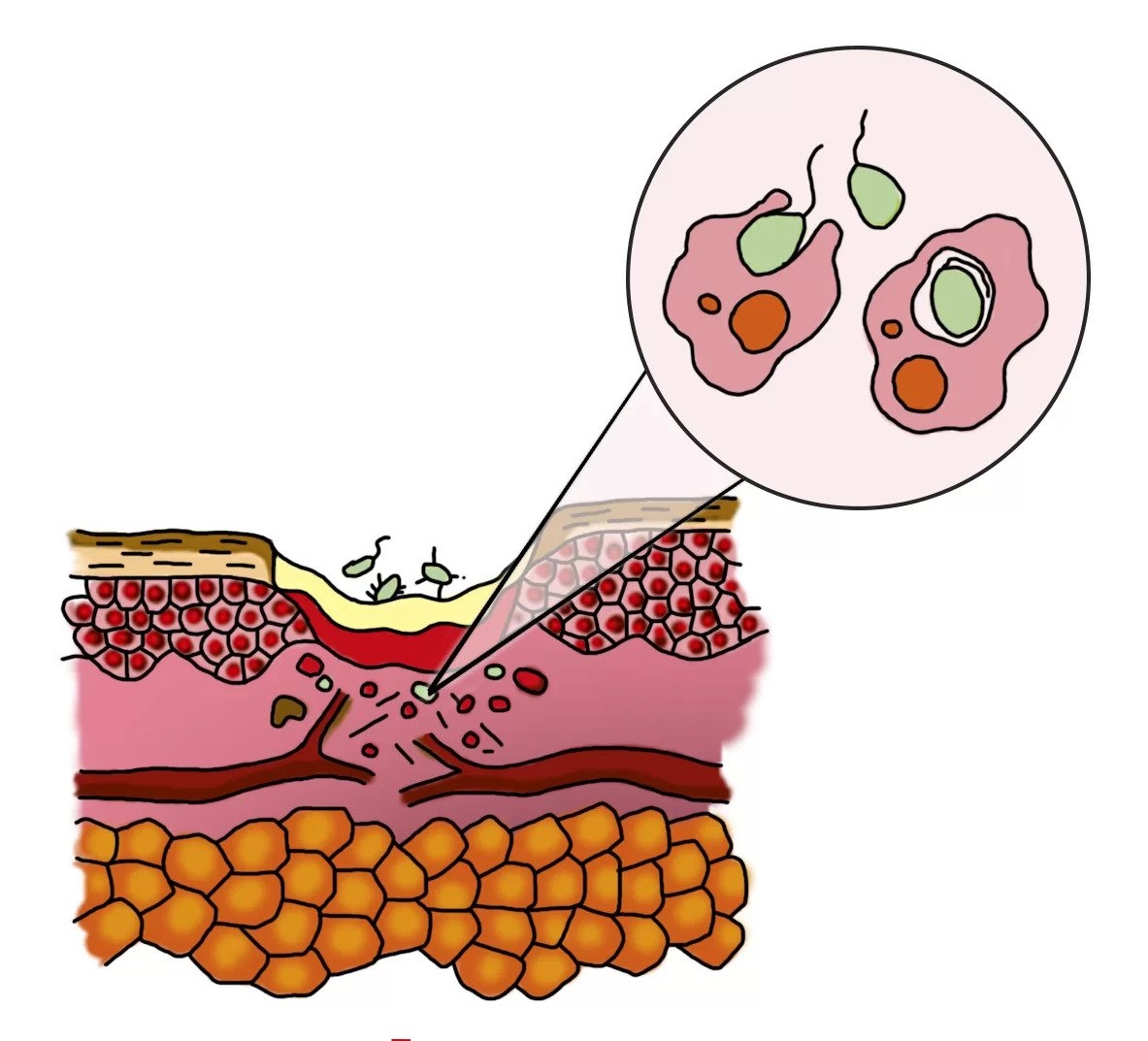
Inflammation phase
- formation of a zone of necrosis
- the appearance and growth of the number of bacteria in the wound

Regeneration phase
- formation of granulation tissue, blood vessels
- epithelialization
In a wound with biofilm
- biofilm formation by bacteria
- migration of phagocytes to the area of inflammation
- cleansing and sterilization of the wound by phagocytes DOES NOT OCCUR.
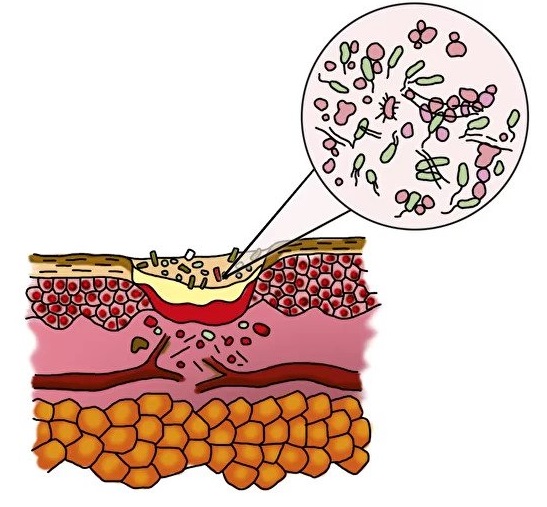
Inflammation phase
- formation of a zone of necrosis
- the appearance and growth of the number of bacteria in the wound
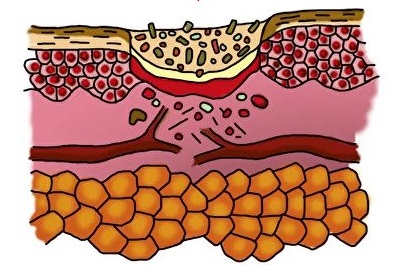
Inflammation phase
- an increase in the zone of necrosis
- an increase in the number of bacteria in the wound
- the regeneration phase DOES NOT COME
wound surface
phagocytes (cells of the immune system)
blood vessels
epithelial cells
separately floating bacteria
dormant bacteria

- In the early stages of the wound process, Entomix® prevents the formation of biofilms by bacteria
- At the later stages, Entomix® leads to the destruction of already formed biofilms, prevents their re-formation
- It reduces the incidence of infectious complications
- It is easily applied to the wound surface and prevents the adhesion of the dressing
- Provides moist wound management without obstructing aeration
- Improves aesthetic healing results
FAQ
What is biofilm and antibiotic resistance? How does biofilm reduce the effectiveness of antibiotics?
The World Health Organization calls the problem of resistance of bacterial infections to antibiotics one of the main challenges of modern medicine. Hundreds of new resistant strains are registered every year around the world. The most common pathogens are Escherichia coli, Klebsiella pneumoniae, Staphylococcus aureus and Streptococcus pneumoniae, followed by Salmonella spp.
The uncontrolled use of antimicrobial drugs in medicine and agriculture launched the process of exchange of plasmids between bacteria in the microcosm. A plasmid containing genes for antibiotic resistance is called a “resist”.
And relatively recently, another resistance mechanism was discovered – the state of the biofilm. Biofilm is a complex structure consisting of the extracellular matrix biopolymers (DNA, proteins, polysaccharides) and “embedded” in it bacterial cells:
- in biofilms have analogs vascular, nervous and immune system of a multicellular organism
- 90% by weight of biofilms – a matrix which protects bacteria from antibiotics and the host’s immune system
- The metabolic activity of bacteria in the biofilm is very low.
Since the mechanism of action of most antibiotics is associated with the effect on the metabolism of the bacterial cell, the effectiveness of such antibiotics is predictably reduced when bacterial cells pass into a biofilm state.
The drop in antibiotic efficiency in the case of biofilm can reach several orders of magnitude. In practice, this means that the dose of the antibiotic must be increased, but in some cases this is impossible because such a dose can be toxic to the patient – i.e. the harm from using antibiotics outweighs their benefits.
Biofilms do not respond well to treatment with existing agents and require the search for new solutions in medicine and pharmacy, for example, such as antibiotic synergists, specialized antibiofilm agents, bacteriophages, etc.
Special attention should be paid to the method of treating infected wounds, which allows avoiding the use of antibiotics – biosurgery.
Clinical studies were carried out in St. Petersburg on the basis of two centers – Clinical Hospital No. 122 named. L.G. Sokolov and the N.V. I.I. Janelidze on nosologies: diabetic ulcer, burns and pyoderma. Photographic materials of the research results are available here .
The term of application of the coating of the hydrogel ENTOMIX ® is during the period necessary for the healing of the wound surface and removal of the symptoms of inflammation of the damaged area of the skin.
For example, the results of clinical studies application ENTOMIKS ® on patients with borderline dermal burns skin (I-II degree of ICD-10 or II-IIIa degree of Congress classification XXVII USSR surgeons 1960 YG) 80% of patients epitelizirovalas completely burn wound to the end 7 days.
Biofilms cause 80% of human bacterial infections and are often virtually immune to treatment with modern antibiotics. Most antibiotics lose their effectiveness when exposed to biofilm because they cannot penetrate its matrix. By destroying the biofilm matrix, FLIP ® 7 restores their activity. For example, we experimentally proved that in the presence of FLIP ® 7, the effectiveness of cefotaxime and meropenem, respectively, on E. coli biofilms increased 40 and 188 times, and the effectiveness of amikacin on S. aureus biofilms increased 330 times. This is the amazing property of FLIP ®7 may allow the use of some traditional antibiotics topically (for example, by direct application to the wound) instead of systemic administration and avoid the side effects of systemic use, such as toxicity, destruction of normal microflora, development of resistance in non-target microorganisms, high cost, etc.